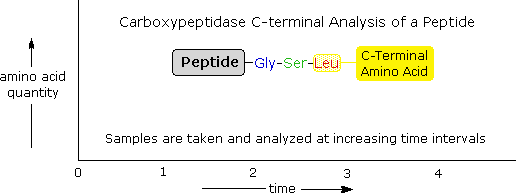
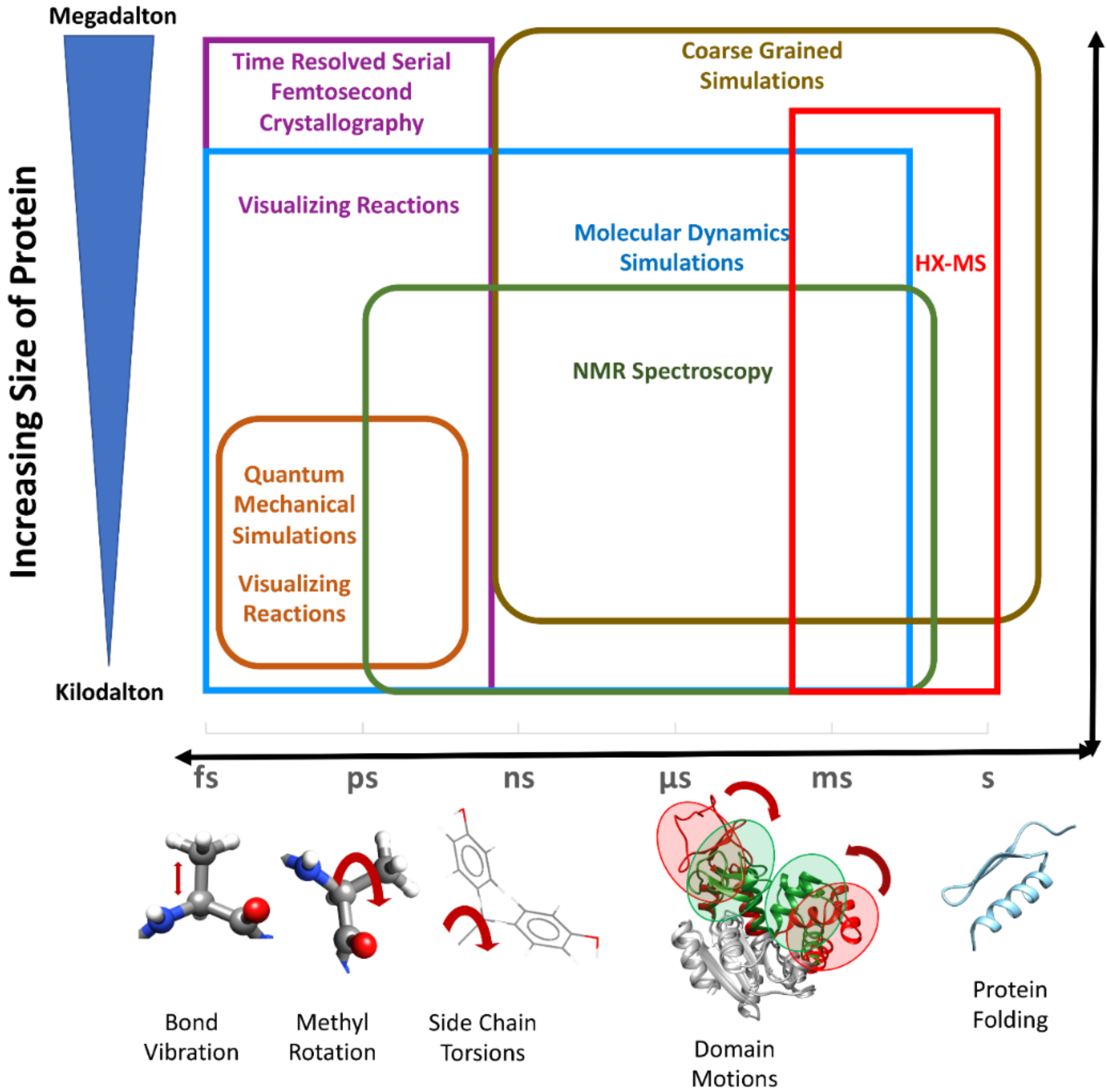
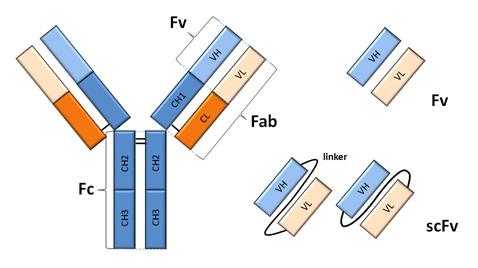
When digesting a pcr fragment make sure to have at least 6 nucleotides between the recognition site and the end of the dna molecule. If the primary structures of these fragments are known it is sometimes possible to deduce part or all of the original structure by taking advantage of overlapping pieces.

Chapter 3 Investigating Proteins Chemistry
What would happen if a protein fragment was not fully. There are two completely different problems that occur in cells when their proteins do not fold properly. Why is my protein fragment being cleavednot fully expressed in r2 e. This does not happen in gametes where transposase is functional. A failure in protein folding causes several known diseases and scientists hypothesize that many more diseases may be related to folding problems. More precisely the sequences that do not contain the motif happen to be aligned either on its right hand side or. Partial hydrolysis will produce a mixture of shorter peptides and some amino acids.
One type of problem called loss of function results when not enough of a particular protein folds properly causing a shortage of specialized workers needed to do a specific job. If donor dna is inserted into the polylinker the enzyme fragment borne on the vector is disrupted no complete β galactosidase protein is formed and the colony is white. Enzymes that help such reactions are called proteases. The restriction enzymes is bound to the substrate dna. Proteasomes are part of a major mechanism by which cells regulate the concentration of particular proteins and degrade misfolded proteins. Add sds 0105 to the loading buffer to dissociate the enzyme from the dna.
A colorless substrate for β galactosidase called x gal is added to the medium and the functional enzyme converts this substrate into a blue dye which colors the colony blue. This test looks for d dimer in the blood. Coli and how do i stop it. From the alignment fourteen blocks are detected as being fully conserved. This protein is not present in germline tissue in somatic cells impaired transposase called a repressor inhibits transposition by binding to itrs. Protein aggregation is one of the most common routes for protein instability and in therapeutic protein production can render the product unfit for release 19in disease states protein aggregation can occur as amyloid fibrillogenesis known to be responsible for neurodegeneration in parkinsons and alzheimers diseases 2021protein aggregation can be reversible or irreversible and the.
Lower the number of units. An unusual type of complementation occurs in which the partial proteins coded by the two fragments unite to form a functional β galactosidase. D dimer is a protein fragment small piece thats made when a blood clot dissolves in the body. Proteasomes are protein complexes which degrade unneeded or damaged proteins by proteolysis a chemical reaction that breaks peptide bonds. The digested dna ran as a smear on an agarose gel. In drosophila somatic cells a special protein inhibits splicing of intron 3 from transposase pre mrna.
If the protein is small and degradation happens during purification you can always try to. Small protein fragments and not just residues can be used as basic building blocks to reconstruct networks of coevolved amino acids in proteins. High levels of d dimer can mean you have a dangerous clotting disorder.